Infection and Local Treatment in Orthopedic Surgery
E. Meani · C. Roman`o · L. Crosby · G. Hofmann (Eds.)
Editorial Coordinator: G. Caloneg
With 173 Figures in 317 Parts and 49 Tables
Editors:
Enzo Meani, MD
Istituto Ortopedico G. Pini
Centro per il Trattamento delle Complicanze
Ortopediche Settiche (COS)
Piazza Cardinal Ferrari 1
20122 Milano, Italy
Carlo Roman`o, MD
Istituto Ortopedico G. Pini
Centro per il Trattamento delle Complicanze
Ortopediche Settiche (COS)
Piazza Cardinal Ferrari 1
20122 Milano, Italy
Lynn Crosby, MD
Department of Orthopaedic Surgery
30 East Apple Street
Dayton, Ohio 45409, United States
Gunther Hofmann, MD
BG-Klinikum Bergmannstrost Halle
Department of Traumatology
Merseburger Stra?e 165
06112 Halle a. d. Saale, Germany
Editorial Coordinator:
Giovanni Calonego
Scientific Coordinator
Tecres S.p.A.
Via A. Doria, 6
37066 Sommacampagna/VR, Italy
Lynn Crosby, MD
Department of Orthopaedic Surgery
30 East Apple Street
Dayton, Ohio 45409, United States
Gunther Hofmann, MD
BG-Klinikum Bergmannstrost Halle
Department of Traumatology
Merseburger Stra?e 165
06112 Halle a. d. Saale, Germany
ISBN 3-540-47998-8 Springer-Verlag Berlin Heidelberg New York
Library of Congress Control Number: 2006939134
This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable for prosecution under the German Copyright Law.
Springer is a part of Springer Science+Business Media
http://www.springer.com
© Springer-Verlag Berlin Heidelberg 2007
Printed in Germany
The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about the application of operative techniques and medications contained in this book. In every individual case the user must check such information by consulting the relevant literature.
Editor: Gabriele Schroder
Desk Editor: Irmela Bohn
Copy-editing: WS Editorial Ltd, Shrewsbury, UK
Production Editor: Joachim W. Schmidt
Cover design: eStudio Calamar, Spain
Typesetting: FotoSatz Pfeifer GmbH, D-82166 Grafelfing
Printed on acid-free paper – 24/3151 – 5 4 3 2 1 0
PMMA as Drug Delivery System and in vivo Release from Spacers
E. Bertazzoni Minelli, A. Benini
Department of Medicine and Public Health, Pharmacology Section, University of Verona
Introduction
Infection is the most serious complication following orthopaedic surgery. The frequency is low, but when present is difficult to treat. Systemic drug administration may not provide inhibitory concentration for a prolonged period, and this is further worsened by the decreased blood supply. The local delivery of antibiotics to the surgical area may may contribute to reduce the infection frequency and the risk of recolonization [16].
In primary prosthetic implants the adequate perioperative antibiotic prophylaxis along with adoption of proper measures of antisepsis seems to be sufficient to contain the risk of infections [10, 19]. 10 % of surgeons utilise antibiotic loaded PMMA in primary hip- or knee- prostheses, increasing to 70 % in the revision surgery in which the risk of septic complications is noticeably increased [10].
The most frequent isolates in orthopaedic surgical infections are Gram-positive cocci. However methicillin-resistant (MRS) and coagulase-negative (CNS) staphylococci are becoming more frequent [35]. In delayed infections (onset 2 to 12 months after prosthesis replacement, according to Cohen classification [10]) CNS and other skin commensals are present and in late infections (onset 12 months after prosthesis replacement) gram-negative and anaerobic bacteria are also isolated [10]. The failure risks are much higher with some “difficult” type of bacteria such as P. aeruginosa, S. aureus (methicillin-resistant), S. epidermidis and Enterococci. Infection commences with adhesion of bacteria to host tissues or prostheses. Biofilm formation, morphological and metabolic modifications (micrococci colonies), combined with reduced host responses in the vicinity of biomaterials, resulting in an active infection around an implant. Organisms within biofilm are difficult to eradicate and will not respond to host defences and antibiotic treatment alone. Removal of the implant, aggressive debridement, local and systemic administration of antibiotics are required [2, 36].
The rationale choice of antimicrobial drugs for local delivery system must take into account the following points: i- microbiological data (micro-organism and its antibiotic susceptibility); ii- bactericidal, wide-spectrum activity of drug; iii- compatibility with cement and other carriers and heat-stability; iv- effects on carrier mechanical resistance, and v- the capacity of elution of the drug from the carrier; vidrug half-life; vii- capacity of inducing hypersensitivity and adverse drug reactions; and finally, viii- antimicrobial activity at the site of infection.
Drug Delivery Systems
The use of bone antibiotic-containing cements (non-degradable polymethylmethacrylate – PMMA –, beads, spacers) and biodegradable systems is increasing and is an adjunct to current therapy (i.e. surgical debridement and systemic antimicrobial therapy) [19].
Biodegradable carriers loaded with different antibiotics are under investigation and are currently being used in some countries, i.e. collagen-gelatin sponge, hydroxyapatite, polymers-polylactide/polyglycolide and polylactate implants- and bioceramics, cancellous bone, calcium phosphate bone substitutes, etc [20]. All the above systems may release antibiotics at concentrations exceeding the MIC for the most common pathogens of prosthetic infections with limited release in systemic circulation and without adverse effects.
The biodegradable or reabsorbable systems can release high local drug concentrations, do not require surgical removal, but may interfere with biological systems and show different interactions with bacteria. The duration of release of antibiotic is dependent on the characteristics of drug carrier.
Two points are essential for the clinical application and satisfactory outcome:
a) Antimicrobial drug pharmacodynamics (on site drug concentration, wide spectrum of activity microorganism susceptibility – or resistance –, tolerability, stability to heat, pH, organic fluids, and presence of necrosis, foreign bodies, etc.).
b) Cement intrinsic characteristics and capacity to release drug (porosity, surface extension, initial drug concentration, thermostability, good capacity to mix with powder);
Moreover, the drug delivery system should not interfere negatively with bone and tissues.
Bone cement mainly consists of PMMA, because of its excellent biocompatibility and ease of manipulation.
Antimicrobial Drugs
Aminoglycosides (gentamicin, tobramycin, arbekacin) are considered to be the antibiotics of choice, because of their wide-spectrum antimicrobial activity, excellent water solubility, chemical and thermal stability, biocompatibility, low allergenicity and low development of resistance during therapy.
However, due to emerging antibiotic resistance there is now a renewed interest for the addition of other antibiotics (vancomycin, clindamycin, fusidic acid, daptomycin, oxazolidinones, fluoroquinolones, peptides, etc.) to drug delivery systems [15, 28]. Vancomycin exhibits positive physico-chemical characteristics similar to those of aminoglycosides, with some limitation regarding the difficulty of polymerization of cement when used in high doses and shorter period of release [9]. Other antibiotics have also been used as additive to PMMA without satisfactory characteristics in vivo. Combinations of antibiotics are also added to bone cements.
The mixing of antibiotics with cement should consider the compatibility of drugs. In Table 1 are summarised the properties of different antibiotics in PMMA. Aminoglycosides and vancomycin show good positive characteristics: antimicrobial activity, adequate release, compatibility and mechanical resistance, excellent tolerability.
The release of other antibiotics such as betalactams (penicillins, cephalosporins, imipenem) is rapid, elevated but limited overtime (48 hours), while rifampicin shows incompatibility with acrylic cement (PMMA) and loss of antimicrobial effect (our personal data [19]). For this purpose, some negative features should be considered, i.e. short half-life, allergenic capacity, and reduced mechanical resistance of cement (beta-lactam agents) or problems with cement incorporation (liquid preparations of gentamicin and clindamycin) or potential negative effects on muscle-skeletal system (fluoroquinolones) [18].
Modifications in antibiotic release should be considered according to bone cement utilised or the combination of two antibiotics, i.e. gentamicin and clindamycin or fusidic acid [28].
Antibiotics utilized for systemic administration are not all useful for drug delivery systems.
PMMA cements impregnated with aminoglycosides and/or vancomycin are currently utilised as local antibiotic carrier in orthopaedic surgical-site infection to treat prosthetic infections (hip, knee, shoulder, etc) [19]. Several experimental models in vitro and in vivo (animal) models have been developed to better understand the release kinetics of different antibiotics from cement and to optimise their use in clinical practice.
Release Kinetics of Drugs from PMMA
The release kinetics from PMMA is similar for different antimicrobial drugs tested. Aminoglycosides and vancomycin elution shows a biphasic (bimodal) profile, consisting of an initial high rapid release of drug followed by a much slower but sustained release. The release of gentamicin and vancomycin is prompt (the maximal drug release from beads occurs within few days) and at inhibitory concentrations which are maintained for 4– 6 weeks [23, 27]. The bone cement PMMA seems a good carrier material for the protracted release of antibiotic drug by diffusion at the site of infection. Gentamicin and vancomycin are still released from explanted spacers after 3– 6 months of implantation; the duration of release is variable [1, 3]. A good inhibitory activity of removed spacers was recorded in vitro for two weeks [21]. The release may be maintained for prolonged periods at inhibitory levels in different preparations. The PMMA cement seems maintain release and kinetics properties similar before and after removal. There is little information on long-term release of antibiotics from impregnated bone cement. Furthermore, gentamicin could still be found in tissue surroundings the implant for over five-ten years. This was though to confer long-term protection against haematogenous infections [39]. Gentamicin was detected in joint fluids of patients up to 10– 20 years following primary hip or knee arthroplasty, using gentamicin-impregnated cement. Concentrations ranged from 0.06 mg/l to 0.85 mg/l (13/25 patients), while only 1 patient showed infection. Data show that gentamicin is present at significant concentrations and in active form inside the cement for a number of years [13, 31].
Variability of Antibiotic Release
This well-known profile of drugs release kinetics from PMMA may present great variability in terms of drug amounts eluted and modality of elution. There is a great variability in bone cement composition (different components), preparations modalities
–i.e. under vacuum or not, hand-made or industrial products-, viscosity and technical characteristics among different brands, different concentrations of antibiotic. Industrial preparations (bone cement, beads and spacers) are considered superior to hand-made preparations because of uniform mixing and standardized procedures [19, 24, 28, 34]. All these factors contribute to variability in drug release and the comparison of data and interpretation of data is very difficult.
Initial drug concentration, cement surface area and porosity are important factors in determining the amount of drug released from beads and spacers [37]. Different mechanisms such as diffusion from cement, surface area and/or cracks and voids in the polymer matrix and bulk porosity are involved in antibiotic release from cement [32]. The release mechanisms are poorly defined and difficult to control. The topic is still debated. The amount of released antibiotic is directly related to cement porosity [37]; the antibiotic release rate of an antibiotic-loaded calcium phosphate bone cement almost tripled on increasing the porosity from 38 % to 69 % [6].
The amount of antibiotic released from cement is different among similar bone cements (gentamicin diffuses from Palacos in larger amounts and for longer period than from Simplex and CMV) [19, 27, 28, 30]. The size is other factor of variability. Beads and mini-beads can release high initial concentrations of gentamicin, but larger beads can maintain a sustained release for more prolonged period in comparison to mini-beads [23, 29].
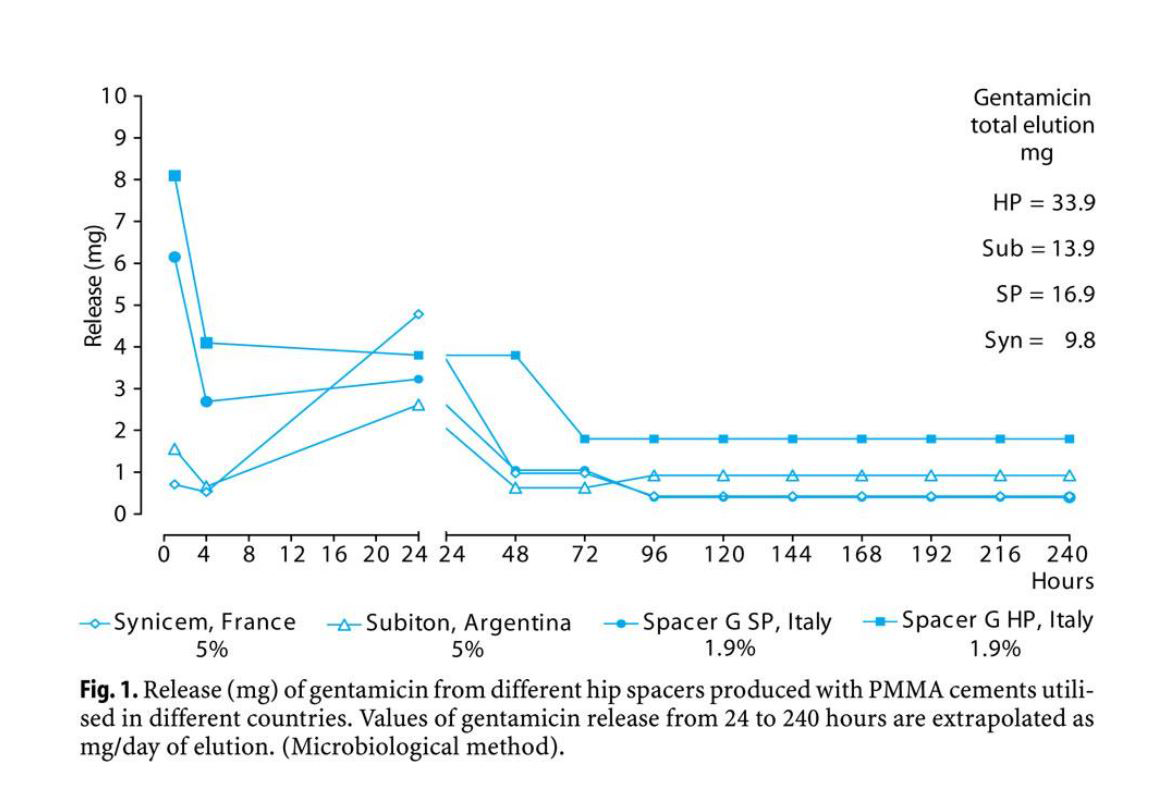
In our experience, we observed a large variation in gentamicin release from different spacers in vitro (Fig. 1). The amounts of gentamicin released from spacers produced in France and in Argentina are lower than those released from spacers produced in Italy (Spacer-G & Spacer-K, Tecres Spa, Italy) in spite of higher content of drug. Spacers may present different release according to porosity: spacer HP can release levels of gentamicin (mg 33.9) higher than those of previous models (SP, mg16.9). Moreover, the kinetics of gentamicin release is very different in the first 24 hours of elution: two spacers release in vitro very low amounts of drug (1.0 mg and 1.8 mg from Argentina and France products, respectively) after 1 and 4 hours of elution, increasing adequately after 24 hours of elution. This kinetics profile is quite different from that described for aminoglycosides from PMMA cement characterised by a peak release in the first 4 hours of elution. The low initial release of antibiotic can contribute to unsatisfactory antimicrobial effect and risk of selection of resistant bacteria. A high release of antibiotic in the first hours of implant should be considered an important factor in order to reduce the risk of selection of resistant bacteria and biofilm production.
Gentamicin-loaded PMMA constitutes an effective drug delivery system for local antibiotic therapy in bone and soft-tissue infections, and gentamicin concentrations at the site of infection can exceed the MIC for the infecting organisms.
Clinical results report similar rate of eradication of infection for beads and spacers. The comparison of beads (70 patients) versus spacers (58 patients) showed that, the rate of eradication of infection was similar between the groups, with an overall rate of 95.3 % [17]. However, the spacer provided higher hip scores, a reduced hospital stay and enhanced functions between stages. The clinical use of spacers in the hip and knee to help eradicate deep infections is increasing [33, 34].
Concentrations and Antimicrobial Activity of Gentamicin and Vancomycin in vivo at the Site of Infection
The release of aminoglycosides and vancomycin from PMMA cement at site of infection seems prompt and effective, presenting high local concentrations above the susceptibility of bacteria and low systemic levels. High intersubject variability was
observed, depending on other additional factors such as fluid presence and output, drainage, vascularization, inflammation, repair tissue, and limb mobilisation [7, 8].
Vancomycin release seems more difficult and shorter than that of gentamicin requiring higher drug concentration in cement [7, 9].
Results exhibit high variability among different preparations and assays rendering it difficult to compare data [4, 10]. Combining two antibiotics in bone cement is a common clinical practice, because of increase in gentamicin resistance among bacterial strains responsible for orthopaedic infection. Vancomycin concentrations in drainage fluid following local administration are higher than those obtained with systemic administration and are superimposable to gentamicin release kinetics. The aminoglycosides and vancomycin in combination show synergistic antimicrobial activity [14, 22]. The use of antibiotic in combination requires additional studies. In vitro studies showed a reduced release of vancomycin when mixed with gentamicin [5], therefore vancomycin was utilized on the surface of spacer w ith gentamicin allowing adequate inhibitory concentrations of antibiotics. This activity was maintained also after removal in the majority of spacers [21]. The superficial application of vancomycin seems to favour high concentrations of antibiotic at the site of infection and allows to optimise the antimicrobial drugs selection according to micro-organisms susceptibility and clinical requirements, and “last minute” application maintaining an adequate capacity of antibiotic release from cement. Nonetheless, the preparation of cement in operating room presents the described limitations, i.e unknown mechanical performances and unpredictable antibiotic
release.
Antimicrobial Activity at the Site of Infection
In addition to the amount of drug released, another point to be considered in the final drug profile evaluation for an adequate orthopaedic infection treatment is the antimicrobial activity of antibiotics at the site of implant, i.e. in the presence of foreign bodies, fibrin, necrosis, fluids secretion, cellular component (PMNs – granulocytes), different pH, blood perfusion, tissues conditions, and oxygen concentration. pH may modify the antimicrobial activity of some antibiotics.
The gentamicin and vancomycin concentrations achieved at the site of infections are far above the MICs for most common pathogens in orthopaedic infections, in spite of the low percentage of release; systemic levels (serum, urine) are low. The risk of adverse reactions, both local at infection site (hypersensitivity or tissue reactions) and systemic, is very low.
However, biomaterials associated infections are an increasing problem. Major concern is about the bone cement utilised for prostheses fixation in primary implants for infection prophylaxis. The long-term exposure to sub-inhibitory concentrations of antibiotics from cements can increase the risk of resistance [13,29].
Biofilm and micrococci are present in removed beads after 14 days, beside of high gentamicin release [29] 18/21 patients showed gentamicin-resistant cocci, but 12/18 were free from infection (tissue samples negative). S. aureus shows different capacity in biofilm formation and for different PMMA cements [28, 29, 38]. In two-stage revision, the implant of temporary spacers and beads with antibiotic loaded cements has proved effective and the problem of resistance is minimal because of the implant removal after a certain time; in addition, spacers perform both mechanical and biological (antimicrobial) objectives.
The problem of resistant bacteria, in presence of local delivery of antibiotics at sub-inhibitory concentration or in presence of biofilm and inflammation products, remains actually unresolved. However, initial high local concentrations of antibiotics are bactericidal and should reduce the risk of selection of resistant bacteria. Routine prophylactic use of antibiotic loaded cement remains a subject of controversy and should be restricted to at risk patients. However, the efficiency of gentamicin-loaded cement in THA along with systemic antimicrobial therapy is confirmed, but the risk of selecting bacteria needs to be considered [11, 12].
In vivo Release from Spacers
There is little data available in the literature on antibiotic release in vivo [4, 7, 14, 21] at the time of prosthesis implantation as well as after removal. Moreover the high variability in results make the comparison of studies difficult. Another question is related to the evaluation of the effective concentrations and antimicrobial inhibitory activity of antibiotics in the infection site and the duration of the effect.
We determined the concentrations of antibiotics at the prosthetic site after temporary spacer implantation in two-stage revision for infected arthroplasty (hip and knee) and evaluated the inhibitory activity of drainage fluids against clinical isolates [4]. The investigation was carried out in collaboration with the Orthopaedic Clinic of our University Hospital.
Samples from serum and fluids from drainage were collected 1, 4 and 24 hours after implantation from ten patients undergoing two-stage revision surgery with a temporary antibiotic loaded spacers (hip or knee, Spacer-G and Spacer-K, respectively) and treated according to microbiological data. Currently, the use of spacers is combined with systemic therapy for prosthetic infections treatment. Vancomycin was given intravenously (1g, bid) or locally in PMMA cement (2.5– 5 %).
Antibiotic concentrations were determined by fluorescence polarisation immunoassay. The antimicrobial activity of gentamicin and vancomycin in drainage fluids activity was determined as bactericidal titer [25], against multiresistant Gram-positive cocci, P. aeruginosa and E. coli.
The results obtained in 3 representative patients are reported in Fig. 2. The release of gentamicin from PMMA cement at site of infection seems prompt and effective, presenting high local concentrations (range 40– 100 mg/L) in the first 24– 48 hours after spacer implant. The concentrations are largely above the susceptibility of bacteria. Serum levels are low ( ‹ 0.2– 0.8 mg/L).
Parenteral administration of vancomycin (1 g ? 2) allows good local penetration, showing similar range of concentrations(range 15– 40 mg/L) in serum and in drainage fluids at different sampling time (1– 24 hours).
The local administration of vancomycin (2.5 %) produces high concentrations in surgical site (18.3– 45.0 mg/L) and low serum levels ( ‹ 1 mg/L). Higher vancomycin concentration (5 %) in cement increased local levels of antibiotic (90– 150 mg/L) in drains. Vancomycin concentrations in drainage fluid following local administration are higher than those obtained with systemic administration. The release kinetics of gentamicin and vancomycin in the site of implant is superimposable (Fig. 2), allowing to exert an inhibitory combined effect.
The ratio of gentamicin to vancomycin concentrations in drainage fluids was 3:1 and 2:1, but high intersubject variability was observed, depending on other additional factors of the patient.
Drainage fluids showed good inhibitory activity against multi-resistant clinical isolates (S. aureus, S. epidermidis, S. haemolyticus, E. coli, P. aeruginosa) (Fig. 2). Drain Bactericidal Titer ranged from 1/8 to 1/64 for bacteria difficult to treat, and from 1/128 to 1/1024 for intermediate and susceptible bacteria in samples collected 1 and 24 hours after implant.
Drugs achieved good bactericidal titer for Gram-positive and Gram-negative pathogens with concentrations in drainage fluids above 2 mg/l and 8 mg/l for vancomycin and gentamicin, respectively. Local gentamicin alone shows inhibitory activity against susceptible microorganisms (knee spacer, patient 4). This is an obvious confirmatory result.
However, the inhibitory effect of the drainage fluids shows large intersubject variability depending on microorganism susceptibility and different concentrations of both antibiotics. We need to define if an optimal concentration ratio of gentamicin to vancomycin is necessary.
The gentamicin and vancomycin in combination show synergistic or summatory antimicrobial activity as defined by FIC determination in vitro (Table 2). Treatments were well tolerated. Gentamicin-containing spacers showed satisfactory clinical results at a follow-up of 5– 7 years [26].
These results confirm the release characteristics of gentamicin and vancomycin from PMMA in vivo. The spacers allow to obtain and maintain inhibitory activity for an adequate local treatment of infection. Spacers seem an adequate system for antibiotic delivery in prosthetic infections in two-stage revision for infected Total Hip and Knee Replacements (THR/TKRs). Our results are in accord with recent data [21, 33].
Conclusions
In conclusion the ideal drug delivery system with controlled release of antibiotic is lacking as well as the ideal antibiotic. Different problems must be solved, such as subinhibitory concentrations of antibiotic released from carriers, false negative cultures, ineffective concentrations or activity at site of infection, reduced biocompatibility and mechanical properties and drug utilisation.
Actually the PMMA cement represents the most useful, suitable and studied local antibiotic delivery system in prosthetic infections. The use of antibiotic-containing PMMA cement in the surgery of revision of total hip and knee arthroplasty represents a local antibiotic therapy supplementary to radical debridment and complementary to systemic therapy.
The choice of the appropriate system (cement, beads, spacers, fleeces, or biodegradable carrier) should based on a careful evaluation of risk/benefit and cost/benefit according to different surgical conditions and requests of patient.
References
- Anagnostakos K, Kelm J, Regitz T et al (2005) In vitro evaluation of antibiotic release from and bacteria growth inhibition by antibiotic-loaded acrylic bone cement spacers. J Biomed Mater Res B Appl Biomater 72(2):373– 378
- Berendt RA. (1999) Infections of prosthetic joints and related problems. In “Infectious Disease” (Armstrong D and Cohen J Eds). Mosby, London. Vol. I, Section 2 – p 44.1– 44.6
- Bertazzoni Minelli E, Benini A, Magnan B et al (2004) Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 53(2):329– 334
- Bertazzoni Minelli E, Benini A, Magnan B et al (2005) Release of antibiotics and inhibitory activity in drainage fluids following temporary spacer implants in two-stage revision surgery. J Chemother 17 (Suppl 3):9
- Bertazzoni Minelli E, Caveiari C, Benini A (2002) Release of antibiotics from polymethylmethacrylate (PMMA) cement. J Chemother 14(5):64– 72
- Bohner M, Lemaitre J, Van Landuyt P et al (1997) Gentamicin-loaded hydraulic calcium phosphate bone cement as antibiotic delivery system. J Pharm Sci 86(5):565– 572 10 PMMA as Drug Delivery System and in vivo Release from Spacers 89
- Brien WW, Salvati EA, Klein R et al (1993). Antibiotic impregnated bone cement in total hip arthroplasty. An in vivo comparison of the elution properties of tobramycin and vancomycin. Clin Orthop Relat Res (296):242– 248
- Bunetel L, Segui A, Cormier M et al. (1989). Release of gentamicin from acrylic bone cement. Clin Pharmacokinet 17(4):291– 297
- Chohfi M, Langlais F, Fourastier J et al. (1998). Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 22(3):171– 177
- Cohen J (1999) Management of chronic infection in prosthetic joints. In “Infectious Disease” (Armstrong D. and Cohen J. Eds). Mosby, London. Vol. I, Section 2 – p 46.1– 46.6
- Diefenbeck M, Muckley T, Hofmann GO (2006) Prophylaxis and treatment of implantrelated infections by local application of antibiotics. Injury 37(Suppl 2):S95– 104
- Enges?ter LB, Lie SA, Espehaug B et al (2003) Antibiotic prophylaxis in total hip arthroplasty. Effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacement followed 0– 14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 74(6):644– 651
- Fletcher MAD, Spencer RF, Langkamer VG et al (2004) Gentamicin concentrations in diagnostic aspirates from 25 patients with hip an knee arthroplasties. Acta Orthop Scand 75(2): 173– 176
- Gonzales Della Valle A, Bostrom M, Brause B et al (2002) Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop Scand 72(3): 237– 240
- Hall EW, Rouse MS, Jacofsky DJ et al (2004) Release of daptomycin from polymethylmetacrylate beads in a continuous flow chamber. Diagn Microbiol Infect Dis 50(4):261– 265
- Henry SL, Hood GA, Seligson D (1993). Long-term implantation of gentamycin-polymethylmetacrylate antibiotic beads. Clin Orthop Relat Res (295):47– 53
- Hsieh PH, Shih CH, Chang AD (2004) Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg (Am) 86(9):1989– 1997
- Huddleston PM, Steckelberg JM, Hanssen AS et al (2000). Ciprofloxacin inhibition of experimental fracture healing. J Bone Joint Surg (Am) 82(2):161– 173
- Joseph TN, Chen AL, Di Cesare PE (2003) Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg 11(1):38– 47.
- Kanellakopoulou K, Giamarellos-Bourboulis E (2000) Carrier system for the local delivery of antibiotics in bone infections. Drugs 59(6):1223– 1232
- Kelm J, Regitz T, Schmitt E et al (2006) In vivo and in vitro studies of antibiotic release from and bacterial growth inhibition by antibiotic-impregnated polymethylmethacrylate hip spacers. Antimicrob Agents Chemother 50(1):332– 335
- Klekamp J, Dawson JM., Haas DW et al (1999) The use of vancomycin and tobramycin in acrylic bone cement: biochemical effects and elution kinetics for use in joint arthroplasty. J Arthroplasty 14(3):339– 346
- Klemm K (2001) The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin Microbiol Infect 7(1):28– 31
- Lewis G. and Bhattaram A. (2006) Influence of a pre-blended antibiotic (gentamicin sulfate powder) on various mechanical, thermal, and physical properties of three acrylic bone cements. J Biomater Appl 20(4):377– 408
- Lorian V (1996) Antibiotic in laboratory medicine. Fourth edition. Williams & Wilkins Ed., Baltimore
- Magnan B, Regis D, Biscaglia R, Bartolozzi P (2001) Preformed acrylic bone cement spacer loaded with antibiotics: use of two-stage procedure in 10 patients because of infected hips after total replacement. Acta Orthop Scand 72(6):591– 594
- Masri BA, Duncan CP, Beauchamp CP (1998) Long-term elution of antibiotics from bonecement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 13(3):331– 338
- Neut D, de Groot EP, Kowalski RS et al (2005) Gentamicin-loaded bone cement with clindamycin or fusidic acid added: biofilm formation and antibiotic release. J Biomed Mater Res A 73(2):165– 170
- Neut D, van de Belt H, Stokroos I, et al. (2001) Biomaterial-associated infection of gentami-90 Basic Research and Local Antibiotic Therapy cin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother. 47(6): 885– 891
- Penner MJ, Duncan CP, Masri BA (1999) The in vitro elution characteristics of antibioticloaded CMW and Palacos-R bone cements. J Arthroplasty 14(2):209– 214
- Powles JW, Spencer RF, Lovering AM (1998) Gentamicin release from old cement during revision hip arthroplasty. J Bone Joint Surg (Br) 80(4):607 – 610
- Seeley SK, Seeley JV, Telehowski P et al (2004) Volume and surface area study of tobramycinpolymethylmetacrylate beads. Clin Orthop Relat Res 420:298 – 303
- Takahira N, Itoman M, Higashi K et al (2003) Treatment outcome of two-stage revision total hip arthroplasty for infected arthroplasty using antibiotic-impregnated cement spacer. J Orthop Sci 8(1):26– 31
- Toms AD, Davidson D, Masri BA et al (2006) The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg (Br) 88(2):149– 155
- Tunney MM, Ramage G, Patrick S et al (1998) Antimicrobial susceptibility of bacteria isolated from orthopaedic implants following revision hip surgery. Antimicrob Agents Chemother 42(11):3002– 3005
- van de Belt H, Neut D, Schenk W et al (2001) Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop Scand 72(6):557– 571
- Van de Belt H, Neut D, Uges DRA et al (2000) Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 21(19):1981–1987
- Van de Belt H, Neut D, Uges DRA, et al (2001). Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials 22(12):1607– 1611
- Wahlig H, Dingeldein E (1980). Antibiotic and bone cements. Experimental and clinical long-term observations. Acta Orthop Scand 51(1):49– 56