Antibiotic-loaded bone cement and its widespread use: is it justified?

Giovanni Calonego
TecresSpA – Sommacampagna (VR), Italy




Introduction
Total hip and total knee arthroplasty (THA, TKA) are clinically efficacious and cost-effective interventions with numerous investigators reporting excellent long-term results in terms of reducing pain and improving function and quality of life in patients with debilitating hip and knee disease (Bozic et al., 2009-2010).
Along with a steady rise in the volume of joint prostheses implanted every year worldwide, there has been a rise in revision THA and TKA (OECD, 2014). Among the main reason for THA revision, infection accounts for 12 – 14.8% (NJR 2013; Bozic et al., 2009), while for the TKA the percentage is much higher, 22 – 25.2% (NJR 2013; Bozic et al., 2010). Periprosthetic joint infection (PJI) is therefore among the main reason for revision.
Improved surgical techniques, the adoption of stringent and efficient antiseptic pre-operative and intra-operative procedures and the optimization of peri-operative systemic antibiotic prophylaxis has led to a significant reduction of deep infection and subsequent revision from 5-10% to 1-2% during the last 20 years (AlBuhairan et al., 2008). ALC was introduced in the early 70s by Buchholz (Buchholz et al., 1981) with the aim to reduce PJI.
Nowadays the use of antibiotic-loaded bone cement has greatly increased: it has become standard practice in northern European countries, while in several other European countries its use is still a matter of debate (Randelli et al., 2010). In US, ALC has only been approved in 2003 and, despite the approved intended use would only cover the second stage following a septic revision, ALC use has currently exceeded 25% of the total bone cement market.
Antibiotic in bone cement prevents bacterial adhesion (BertazzoniMinelli et al., 2011): bacterial adhesion is the first step towards bacterial colonization, multiplication and formation of a biofilm. Bacterial growth on implants depends on biofilm production: the higher the roughness of the material, the higher the bacterial adhesion. PMMA is characterized by a rough surface. Bacteria resistance to antibiotic effect and immune host system is 1000 times higher in biofilm (van de Belt et al., 2001). The aim is therefore to avoid to get to this point when biofilm forms, leading to a chronic infection where the implant will have to be removed.
As previously said, the use of ALC in primary prosthesis fixation is still debated, so in order to be able to provide a reasonable and scientific answer to the question “is the widespread use of ALC justified?” we should first answer to a number of other questions: 1) what’s the aim about using ALC; 2) what about ALC success rate on infection eradication; 3) what about the best and safest ALC to use; 4), when should ALC be used; 5) is there any potential detrimental effect?
Aim of use of ALC
The release of antibiotic is the main aim of ALC: in literature high dose (>2g AB per 40 PMMA) and low dose (<2g per 40g PMMA) ALC is described (Jiranek et al., 2006), it is known that vacuum mixing is better than hand mixing in improving the mechanical performances of the bone cement, however antibiotic release may be greatly influenced: if we refer to commercially available ALC different cement will be differently affected by vacuum mixing (Meyer et al., 2011). In principle the reduction of cement porosity will reduce also the antibiotic release potential.
ALC use and infection rate
In terms of prophylaxis, it was demonstrated that the combined use of systemic antibiotic and ALC in THA was producing the lowest risk of revision due to infection (Engesaeter et al., 2003). This information was confirmed in a review paper where it was shown that the use of ALC is associated with a significant reduction (50% less) in the risk of deep infection following THA (Parvizi et al., 2008). However in TKA some recent paper was not able to confirm the same positive results (Namba et al., 2009; Gandhi et al., 2009).
The release of antibiotic from ALC has a well known kinetics, with a peak release during the first days followed by a long tail of lower release. Therefore it is likely that optimal antibiotic accumulation in the wound hematoma would occur if wound drains were avoided or their duration of use minimized. With the presence of drains significant amount of antibiotic are lost (Squire et al., 2008).
Which ALC to use
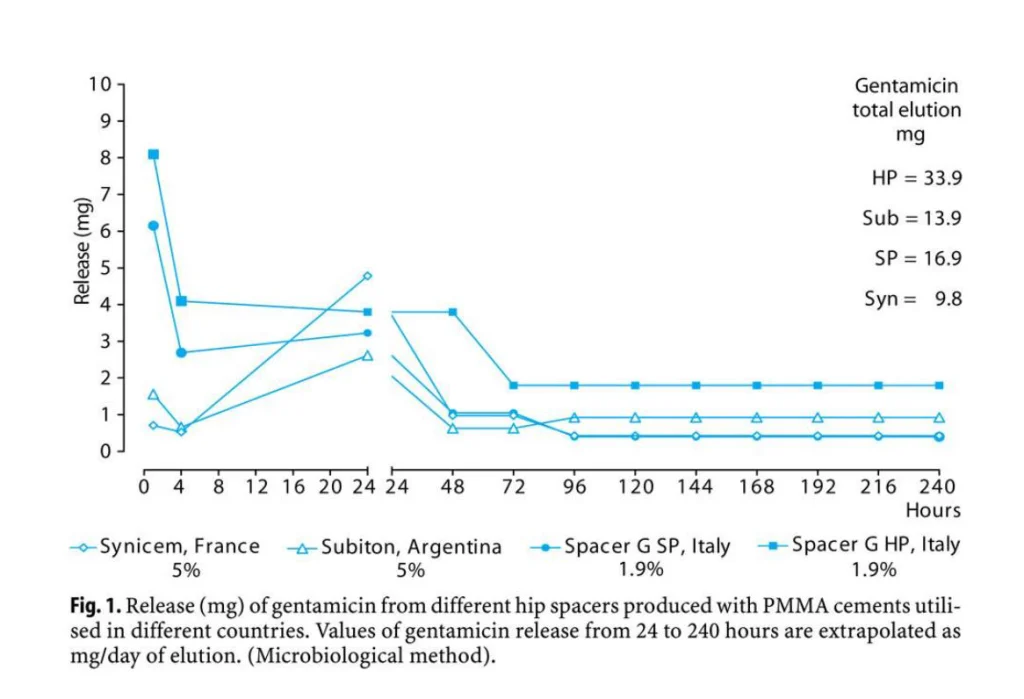
A number of antibiotics (Table 1) may be admixed to bone cement (Gasparini et al., 2014), however commercially available single antibiotic ALC are loaded with an Aminoglycoside (Gentamicn or Tobramycin). Different cements shows different release, even though the same antibiotic is used: results depends on the properties of each cement (Squire et al., 2008). The choice of adding an Aminoglycoside (Gentamicin) was based on release studies which demonstrated that among antibiotics Gentamicin was showing the best elution performances (Wahlig et al., 1980) from PMMA, the most extended spectrum of activity and no negative effect on mechanical performances.
Table 1
| Amikacinsulphate | Ceftriaxonesodium |
| Gentamicinsulphate | Clindamycinphosphate |
| Streptomicinsulphate | Colistinsulphate |
| Tobramycin sulphate | Meropenem |
| Teicoplanin | Rifampicin* |
| Vancomycin hydrochloride | Tigecycline |
*Bone cement does not polymerize
When to use ALC
Nowadays some concern have arisen for the increase in gentamicin resistance, however the use of gentamicin bone cement for prophylaxis appears justified. Most published papers have shown a positive effect in the use of ALC in terms of infection rate reduction, even though some authors do not agree with the use in primary arthroplasty, unless we are speaking of high risk patients or joint arthroplasty revision (Bistolfi et al., 2011).
Any potential detrimental effect
As regards possible problems associated with the presence of antibiotic in bone cement the following should be considered: systemic toxicity, hypersensitivity reaction, mechanical hazards, bacterial resistance hazards and cost rising. The discussion will be limited to commercially available ALC.
The risk of systemic toxicity is not likely. Toxicity can be dosage or antibiotic dependent. If we refer to gentamicin, diffusion in the bloodstream is minimal (although high concentration may be achieved locally), the risk of hearing loss and renal failure, which is cumulative, cannot be associated to the low serum concentration determined by ALC, while it can be associated to previous doses received within the last few months or years. Eventually acute or chronic kidney injury (AKI, CKI) occur in patients with previous kidney or liver disfunction(Passuti et al., 2003).
The evidence for hapten mediated hypersensitivity to the interaction of antibiotics and bone cement is very limited (Passuti et al., 2003); gentamicin sensitization may result from occupational exposure to gentamicin containing bone cements or from systemic medication with aminoglycosides (Liippo et al., 2008).
All ALC on the market fulfill the basic mechanical requirements (ISO Standard 5833), although there are significant difference in mechanical performance (Kuehn et al., 2005) among cements. It shall be underlined that no clinical study has proved that a mechanically stronger cement will produce better clinical outcomes.
As concerns bacterial resistance, apparently there is no direct relationship between the use of gentamicin-loaded cement and bacterial resistance increase. In Northern Europe the use of gentamicin-loaded cement in primary prostheses has reached 100%, however according to the European Antimicrobial Surveillance Network (ECDC Surveillance Report, 2013) the rate of resistance in these countries is the lowest in Europe.
In any case the use of Vancomycin cement as a primary agent in prophylaxis should be avoided because of the emergence of resistant bacteria and the need to reserve this antibiotic for those patients which require vancomycin for treatment purposes (Hanssen et al., 2004).
Eventually, the increasing use of ALC has lead to the increase of costs. This is more relevant in US, where ALC is 3 times more expensive compared to plain bone cement. It was shown that while in primary arthroplasty it increases the overall costs, in revision due to infection the use of ALC was found to have an incremental cost-effectiveness ratio of $37,355 per QALY, with a cost decreasing of $650 per patient (Cummins et al., 2009).
Conclusion
In conclusion, the use of ALC must be considered as a support strategy in prevention and not the solution of infections; in primary implants, ALC is justified only in high risk patients; it is recommended for revisions; the perfect antibiotic in ALC does not exist; avoid using Vancomycin as first step; ALC is successful only if associated with systemic antibiotics; use of ALAC significantly rises cost-effectivness in implant revision.
References:
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br. 2008 Jul;90(7):915-9. doi: 10.1302/0301-620X.90B7.20498.;
- Bistolfi A, Massazza G et al. Antibiotic-loaded cement in orthopedic surgery: a review. ISRN Orthop. 2011 Aug 7;2011:290851. doi: 10.5402/2011/290851. eCollection 2011. Review.;
- Bozic KJ, Kurtz SM et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009 Jan;91(1):128-33. doi: 10.2106/JBJS.H.00155.;
- Bozic KJ, Kurtz SM et al. The epidemiology of revision total knee arthroplasty in the United States. ClinOrthopRelat Res. 2010 Jan;468(1):45-51. doi: 10.1007/s11999-009-0945-0. Epub 2009 Jun 25.;
- Buchholz HW, Elson RA et al. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63-B(3):342-53.;
- Cummins JS, Tomek IM et al. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009 Mar 1;91(3):634-41. doi: 10.2106/JBJS.G.01029.;
- ECDC Surveillance Report, 2013. http://www.ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2013.pdf;
- Engesaeter LB, Lie SA et al. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. ActaOrthop Scand. 2003 Dec;74(6):644-51.;
- Gandhi R, Razak F et al. Antibiotic bone cement and the incidence of deep infection after total knee arthroplasty. J Arthroplasty. 2009 Oct;24(7):1015-8. doi: 10.1016/j.arth.2008.08.004. Epub 2008 Sep 26.;
- Gasparini G, De Gori M et al. Drug elution from high-dose antibiotic-loaded acrylic cement: a comparative, in vitro study. Orthopedics. 2014 Nov 1;37(11):e999-e1005. Doi: 10.3928/01477447-20141023-57.;
- Hanssen AD. Prophylactic use of antibiotic bone cement: an emerging standard–in opposition. J Arthroplasty. 2004 Jun;19(4 Suppl 1):73-7.;
- Health at a glance: Europe 2014 OECD. Hip and Knee Replacement. http://ec.europa.eu/health/reports/european/health_glance_2014_en.htm;
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006 Nov;88(11):2487-500.;
- Kuehn KD, Ege W, Gopp U. Acrylic bone cements: mechanical and physical properties. OrthopClin North Am. 2005 Jan;36(1):29-39, v-vi.;
- Liippo J, Lammintausta K. Positive patch test reactions to gentamicin show sensitization to aminoglycosides from topical therapies, bone cements, and from systemic medication. Contact Dermatitis. 2008 Nov;59(5):268-72. doi: 10.1111/j.1600-0536.2008.01419.x.;
- Meyer J, Piller G et al. Vacuum-mixing significantly changes antibiotic elution characteristics of commercially available antibiotic-impregnated bone cements. J Bone Joint Surg Am. 2011 Nov 16;93(22):2049-56. doi: 10.2106/JBJS.J.01777.;
- Namba RS, Chen Y et al. Outcomes of routine use of antibiotic-loaded cement in primary total knee arthroplasty. J Arthroplasty. 2009 Sep;24(6 Suppl):44-7. doi: 10.1016/j.arth.2009.05.007. Epub 2009 Jul 4.;
- Parvizi J, Saleh KJ et al. Efficacy of antibiotic-impregnated cement in total hip replacement. ActaOrthop. 2008 Jun;79(3):335-41. Doi: 10.1080/17453670710015229.;
- Passuti N, Gouin F. Antibiotic-loaded bone cement in orthopedic surgery. Joint Bone Spine. 2003 Jun;70(3):169-74.;
- Randelli P, Evola FR et al. Prophylactic use of antibiotic-loaded bone cement in primary total knee replacement. Knee Surg Sports TraumatolArthrosc. 2010 Feb;18(2):181-6. doi: 10.1007/s00167-009-0921-y. Epub 2009 Oct 1.;
- Squire MW, Ludwig BJ et al. Premixed antibiotic bone cement: an in vitro comparison of antimicrobial efficacy. J Arthroplasty. 2008 Sep;23(6 Suppl 1):110-4. doi: 10.1016/j.arth.2008.03.014. Epub 2008 Jul 9.;
- van de Belt H, Neut D et al. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. ActaOrthop Scand. 2001 Dec;72(6):557-71. Review.;
- Wahlig H, Dingeldein E. Antibiotics and bone cements. Experimental and clinical long-term observations. ActaOrthop Scand. 1980 Feb;51(1):49-56.