Bone cement and cementation technique, antibiotics and bone cement spacers: what you should know
Giovanni Calonego, BS – Scientific Coordinator, TecresSpA (Italy)

Acrylic bone cement has been used successfully in orthopaedic practice for more than 50 years, thanks to the pioneering work of Sir John Charnley in the late 50s (Charnley, 1972).The great results achieved by Charnley with the low friction arthroplasty open the way to the work of Buchholz that, in the early 70s,realized that bone cement could be a great material also for the delivery of drugs thus helping in the reduction of the infection rate (Buchholz, 1981).
The evolution of the cementing technique (Breusch – Malchau, 2005) and of the implant design (Scheerlink, 2006) has been the key for the success of the cemented fixation. From finger packing, with minimal pressurisation cement contamination risk (air, fat, blood), cementing technique evolved through the use of cement gun, endomedullary plug and pressurizer to improve cement penetration and inter-digitation. The next step was the introduction of mixing systems, a better bone bed preparation and the use of pulsed lavage in an effort to improve cement performances to guarantee along-lasting outcome (third generation cementing technique). The use of centralisersdidfurtherly improve cement performances, allowing for even cement and stress distribution (Berger, 1997).
Implant design evolution has followed two distinct philosophies: load tapers or force-closed femoral stems, designed to subside (Exeter stem, CPT stem, C-stem) and composite beam or shape-closed designs, designed not to subside (Charnley Elite, Lubinus SP2). Other important features were related to surface finish (polished or smooth / roughened) and stem geometry design (overall shape, straight or anatomical; cross-section, oval or square; collar presence; tip shape; stem length) (Scheerlink, 2006).
The combined evolution of cementing technique and implant design has been shown to produce excellent long-term outcome of cemented implants with a survival rate exceeding 97% at 10 years (Swedish Hip Register Report, 2004 & 2012).
According to recent estimate (Millennium Group Survey, 2011) in 2012 more than 3 million hip and knee replacements have been performed in 9 markets (UK, France, Germany, Italy, USA, Japan, Brail, China, India) and worldwide it is estimated that this number has reached some 4.5 million hips and knees. Most of these implants are fixed using bone cement, with or without antibiotic. In Ukraine the number of hip and knees has been growing steadily, and in 2012 about 7000 implants have been made.
The success and diffusion of arthroplasty do not come without problems. Revision arthroplasty is a great problem which increases the healthcare costs, and in specific revision for infection is a major problem. According to recent reports infection is among the three main reason for revision exceeding 20% (Bozic, 2010; NJR 2011). All in all infection following a primary implant exceeds 2% over a 10 year period (Ong, 2009; Kurtz, 2010). A higher rate instead is encountered when infection occurs to a revision implant.
In an effort to reduce the risk of infection, the use of antibiotic-loaded acrylic cement (ALAC) has become quite common. However not all drugs can be added to bone cement. As a general rule antibiotics shall be thermostable, water soluble, achieve a bactericidal effect. In addition they should be gradually released, with a limited inflammatory /allergic potential and not compromising the mechanical performances of bone cement (Joseph, 2003). The industry has then further requirements, as the antibiotic shall be available in bulk, in powder form and shall be stable to sterilization and ageing. In addition the Regulatory approval path is quite long and expensive. Therefore the high costs involved in research and development, and registration along with the request for low prices is a terrible brake for the introduction of new products (ALAC).
The success and diffusion of arthroplasty do not come without problems. Revision arthroplasty is a great problem which increases the healthcare costs, and in specific revision for infection is a major problem. According to recent reports infection is among the three main reason for revision exceeding 20% (Bozic, 2010; NJR 2011). All in all infection following a primary implant exceeds 2% over a 10 year period (Ong, 2009; Kurtz, 2010). A higher rate instead is encountered when infection occurs to a revision implant.
In an effort to reduce the risk of infection, the use of antibiotic-loaded acrylic cement (ALAC) has become quite common. However not all drugs can be added to bone cement. As a general rule antibiotics shall be thermostable, water soluble, achieve a bactericidal effect. In addition they should be gradually released, with a limited inflammatory /allergic potential and not compromising the mechanical performances of bone cement (Joseph, 2003). The industry has then further requirements, as the antibiotic shall be available in bulk, in powder form and shall be stable to sterilization and ageing. In addition the Regulatory approval path is quite long and expensive. Therefore the high costs involved in research and development, and registration along with the request for low prices is a terrible brake for the introduction of new products (ALAC).
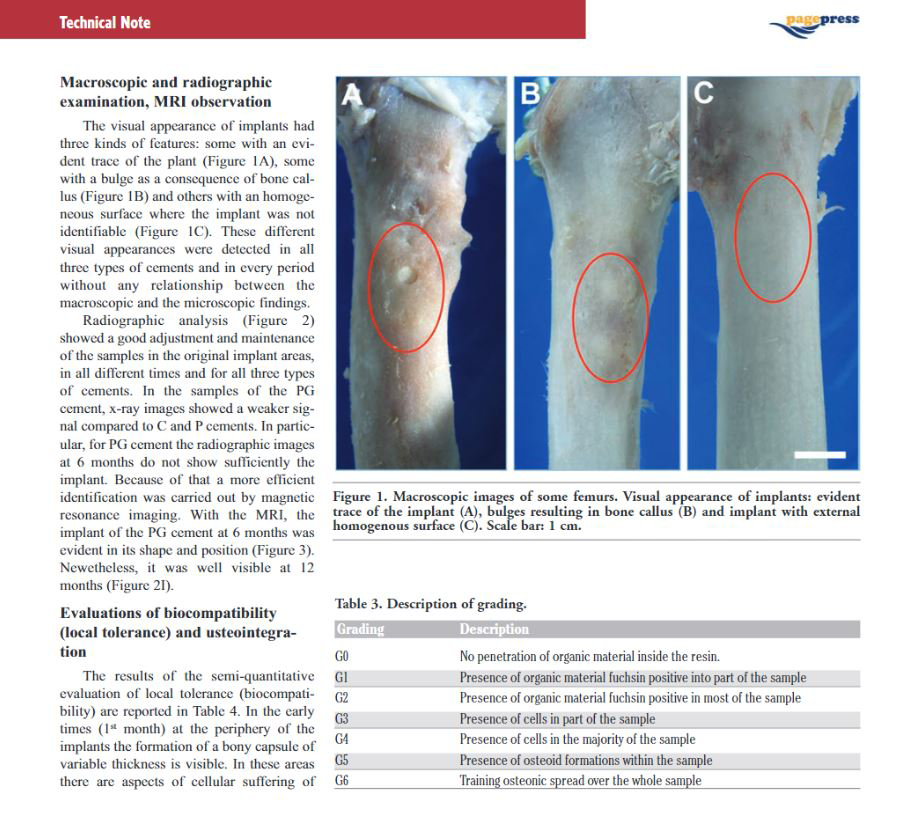
The theory on antibiotic release from cement is still under debate, some authors favour a diffusion theory, however the majority supports the idea that release is mainly a surface phenomenon. Release is a function of liquid penetration into the PMMA matrix. As PMMA is not a porous structure, it is clear that release will be related to bulk porosity, and influenced by surface rugosity, presence of interconnecting pores and cracks. In relation to these properties we will have low and high porosity bone cements with different release performances (van de Belt, 2000).
The local release of antibiotics from PMMA is quite interesting as the antibiotic is directly delivered into the operative site, attaining very high local concentrations without any systemic side effect (Bertazzoni Minelli, 2004).
Currently, commercially available ALACs include cement with Gentamicin or Tobramycin, and cement with Gentamicin+Clindamycin, Erhytromycin+Colistin, Gentamicin+Vancomycin. Single antibiotic cement is generally used with patient at risk, but in a number of countries (Northern Europe) it is used for the fixation of primary implants as a prophylactic means to reduce infection (Espehaug, 1997). Double antibiotic cement is instead used in established infections in one- or two-stage procedure. In two-stage procedures ALAC spacers are used. Industrially preformed ALAC spacers are also available loaded with Gentamicin or Gentamicin+Vancomycin.
The use of ALAC should be done with caution in order not to increase bacterial resistance. The most commonly used ALACs are single antibiotic cements, loaded with Gentamicin or Tobramycin. Both antibiotics belong to the Aminoglycosides family and share the same spectrum of activity without any clinical difference in terms of results achieved. ALAC use is not uniform across the world: in Mediterranean Europe ALAC is only reserved for risk patients or in revision cases, while in Northern Europe ALAC is used for the fixation of primary implants (see Swedish, Norwegian, UK and German Registers). In US, ALAC cement should only be reserved for the second stage following a septic problem, however recent reports indicates that the use is reaching 50%. On the contrary in Japan commercial ALAC is not approved, and is prepared in the OR by the surgeon.
Tecres is an Italian company which manufactures acrylic bone cement products and is present in the market for more than 25 years. In 1986 the first bone cement was launched (Cemex Standard), which was radiotransparent, quickly followed by the radiopaque version (Cemex RX). Soon after a high viscosity version (CemexIsoplastics) was introduced. In 1991 a great innovation was made by introducing the first bone cement inside a syringe device (Cemex System, Figure 1), which serves as a mixing and delivery device, preventing from microbial contamination and MMA fumes.The first antibiotic-loaded version was introduced in 1996 (CemexGenta), and recently the innovative and unique double-antibiotic version including Gentamicin and Vancomycin was launched (Vancogenx).
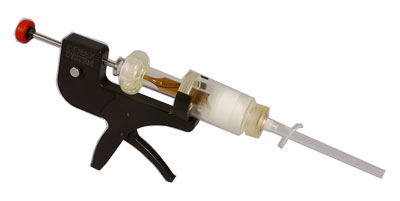
Figure 1. Cemex System bone cement and Gun. Powder and liquid are contained in the syringe.
The unicity of Tecres bone cement comes from the formula, and in particular from the Cemex PMMA powder which, being uniform and round, allows to reduce the amount of liquid monomer (MMA) required providing the cement great advantages. Compared to competitor products, Cemex has around 30% less liquid MMA (Figure 2).

Figure 2. Comparison of MMA ampoules of common bone cements (Palacos R – Heraeus, Simplex P – Stryker; SmartSet-Depuy; Cemex RX – Tecres).
MMA affects the properties of bone cement. In particular MMA generates 130cal/g, therefore the more the liquid the more the energy (heat) generated. This is the theory, which has been confirmed by testing Cemex in comparison with other cements. The uniform and round shape of the Cemex PMMA beads has allowed to reduce the MMA amount, making the cement more compact and less porous. So the MMA reduction is key to the advantages of the Cemex bone cement line: lower porosity; lower polimerisation temperature; lower MMA release; lower shrinkage.
Chemico-physical, mechanical and clinical testing have been performed in the most important Mechanical and Orthopaedic Institutes and Research Centers in Europe and US to assess and conform the performances and quality of the Cemex bone cement line (Milan Polytechnic, Nijmegen Uni, Rizzoli Institutes, Goteborg Uni, Munich Uni, Memphis Uni, Nurnberg Uni…). As a consequence of the compactness of the Cemex powder, vacuum mixing is not necessary to achieve the best performances, as shown by published clinical data (Soderlund, 2012; Dahl, 2012).
The excellent and outstanding features have made Cemex bone cement diffused worldwide in more than 65 countries, including Europe, US, South Korea, Japan, China, India and Australia. In Ukraine Cemex was introduced starting from 2004, while only recently the Gentamicin and Gentamicin+Vancomycin version have been registered.
Release of antibiotic from CemexGenta is comparable or even better than competitors (Squire, 2008), and the best elution is achieved not applying vacuum (Meyer, 2011). The use of vacuum is not needed, as Cemex powder is very compact and vacuum would reduce micro-porosity negatively affecting release.
While chemico-physical and mechanical performances are important for any bone cement, the clinical outcome is key to its success. Cemex clinical performances have been assessed in two RSA studies performed in Sweden and Germany. RSA (radiosteremetricanalysis) is a radiological technique thatenables calculation of the 3D translational and rotational movements of theimplant relative to the bone with high precision andaccuracy and has become the gold standard for clinicalevaluation of new surgical techniques and implants. RSA is part of the recommended stepwise introduction ofnew surgical techniques and implants (Husby, 2010). RSA utilizes modified implants with tantalum markers, and tantalum markers included inside bone and bone cement. Through a double X-ray examination repeated over time and a specific software it is possible to follow the migration of the implant.
RSA studies, which prospective and randomized, generally last 2 years: this is enough to assess the long-term performances of an implant, as the principle of RSA is that if there is a stable interface (bone-bone cement; bone cement-implant) at the beginning, the implant will be stable and will not migrate (Karrholm, 1994).
The first RSA study compares the performances of Cemex and Palacos R(vacuum mixed) used for the fixation of Lubinus SP2 stem and Lubinus cup. At 5 years of follow-up no difference was found in terms of stem migration and head penetration (wear) (Nivbrant, 2001). At 10 years instead the implants fixed with Palacos R showed a larger magnitude of rotation into flexion/extension and retroversion (Soderlund, 2012), while no difference was found in terms of 3D and proximal wear (Dahl, 2012). This means that Cemex bone cement performs as good as Palacos R, and without the application of vacuum.
The second RSA study instead compares the antibiotic version, CemexGenta and RefobacinPalacos RG, used for the fixation of the same implant (Lubinus SP2 stem). At two years of follow-up no difference in terms of stem migration was found between the two groups (Pitto, 2003). The five years follow-up (only patients with implants fixed with CemexGenta) showed the same type of migration found in the first RSA study (Pitto, 2007), thus indicating that Cemex and CemexGenta shares, as expected, the same excellent clinical performances.
These results, along with the solid scientific background, has led to the introduction of Cemex in two very selective markets, Sweden and Norway (as documented in the well-known Registers) were only a few cements are used, i.e. those who has proven clinical effectiveness (Swedish Hip Register, Norwegian Arthroplasty Register).
As previously introduced, the great success of arthroplasty is not without problems. And orthopaedic infection is a major complication. When dealing with a chronic infection, the only way to get rid of infection is to remove the implant. This can be done in one single procedure (one-stage exchange) or in a two-step procedure (two-stage exchange). In either cases, ALAC is used. Two-stage exchange with the use of a spacer is considered the golden standard treatment approach achieving en eradication rate exceeding 90% (Romanò, 2012).
Two-stage exchange foresees a first stage in which a thorough surgical debridement is performed and an ALAC spacer is applied. The first stage is followed by an interim period of 2 – 3 months (or more) during which a targeted systemic antibiotic therapy of 4 – 6 weeks is administered. When the serological markers indicate the remission of the infection and the clinical conditions of the patient are favourable, the second stage is programmed. This consists of ALAC spacer removal, additional surgical debridement and implantation of a definitive implant (Romanò, 2012).
ALAC spacers can be hand-made in the OR or can be premanufactured and ready to use.
Tecres has successfully introduced starting from 1996 the preformed spacers, with standardized mechanical and pharmacological performances. Such devices are available for hip, knee and shoulder prosthesis infection (Figure 3). They have been designed to maintain space and mobility, and allow partial weight-bearing (Baleani, 2003; Villa, 2007; Romanò, 2007; Logoluso, 2011). The mechanical performances are comparable to those of a primary implant (for 6 months), while the pharmacological performances allow for prompt high and effective release (Regis, 2013), with maintainance of high antibiotic concentration over the spacer implantation period (Mutimer, 2010).


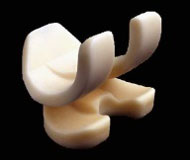

Figure 3. Preformed hip, knee and shoulder spacers
The mechanical performances are key to avoid the common complication which may occur using hand-made spacers, such as spacer breakage (Figure 4), bone defect progression, periprosthetic femoral fracture, dislocation (Jung, 2009). The pharmacological performances instead guarantees an effective and prolonged in situ release of antibiotic, which cannot be guaranteed when making hand-made spacers in the OR (Moojen, 2008; Rogers, 2011).
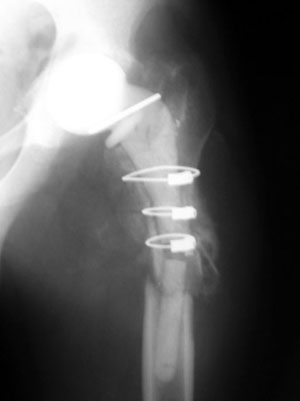
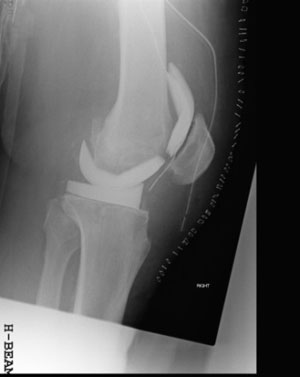
Tecres preformed spacers are available with Gentamicin only (Spacer-G, Spacer-K, Spacer-S) or with the combination Gentamicin+Vancomycin (Vancogenx-Space Hip & Knee). Transparents and Trials are available for the selection of the correct size. The excellent results achieved when using the preformed spacers have been published in multiple papers in peer-reviewed journals. In consideration of the large number of published papers, it is possible to make a systematic review considering only papers with more than 10 patients, with a mean follow-up larger than 1 year and limited to gentamicin spacers (Table 1). Among the 11 papers included in the review, 507 patients from 12 centers are considered. At a mean follow-up of 44 months, the eradication rate was found to exceed 94%.
In conclusion, the evolution of cementing technique and implant design has led to excellent long-term clinical performances of cemented implants. Orthopaedic infection is a major complication: ALAC and ALAC spacers are helpful for solving this relevant problem. Bone cement, introduced in the orthopaedic field more than 50 years ago, can be improved: Cemex is an innovative cement line, clinically reliable. Industrially preformed spacers are safe and effective in two-stage revision, ready to use and able to guarantee a high quality of life to patients.
References#1
- Baleani M, Traina F, Toni A. The mechanical behaviour of a preformed hip spacer. Hip International 2003; 13:159-62
- Berger RA, Seel MJ, Wood K, Evans R, D’Antonio J, Rubash HE.Effect of a centralizing device on cement mantle deficiencies and initial prosthetic alignment in total hip arthroplasty.J Arthroplasty. 1997 Jun;12(4):434-43.
- BertazzoniMinelli E, Benini A, Magnan B, Bartolozzi P.Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty.J AntimicrobChemother. 2004 Feb;53(2):329-34. Epub 2003 Dec 19.
- Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ.The epidemiology of revision total knee arthroplasty in the United States.ClinOrthopRelat Res. 2010 Jan;468(1):45-51. Epub 2009 Jun 25.
- Breusch S, Malchau H. The Well-Cemented Total Hip Arthroplasty. Theory and practice. Springer Verlag, 2005
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A.Management of deep infection of total hip replacement.J Bone Joint Surg Br. 1981;63-B(3):342-53.
- Charnley J.The long-term results of low-friction arthroplasty of the hip performed as a primary intervention.J Bone JointSurg Br. 1972 Feb;54(1):61-76
- Coffey MJ, Ely EE, Crosby LA. Treatment of glenohumeral sepsis with a commercially produced antibiotic-impregnated cement spacer. J Shoulder Elbow Surg. 2010 Sep;19(6):868-73. Epub 2010 Apr 14.
- Dahl J, Söderlund P, Nivbrant B, Nordsletten L, Röhrl SM.Less wear with aluminium-oxide heads than cobalt-chrome heads with ultra-high molecular weight cemented polyethylene cups: a ten-year follow-up with radiostereometry.IntOrthop. 2012 Mar;36(3):485-90. Epub 2011 Aug 26.
- D’Angelo F, Negri L, Binda T, Zatti G, Cherubino P.The use of a preformed spacer in two-stage revision of infected hip arthroplasties.Musculoskelet Surg. 2011 Aug;95(2):115-20. Epub 2011 Apr 9.
- Degen RM, Davey JR, Davey JR, Howard JL, McCalden RW, Naudie DD.Does a prefabricated gentamicin-impregnated, load-bearing spacer control periprosthetic hip infection?ClinOrthopRelat Res. 2012 Oct;470(10):2724-9.
- England, Wales & Northern Ireland National Joint Registry http://www-new.njrcentre.org.uk/njrcentre/Default.aspx
- Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N.Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995.J Bone Joint Surg Br. 1997 Jul;79(4):590-5.
- García-Oltra E, Bori G, Tomas X, Gallart X, Garcia S, Soriano A.Radiological evaluation of acetabular erosion after antibiotic-impregnated polymethylmethacrylate spacer (Spacer-G).J Arthroplasty. 2013 Jun;28(6):1021-4. Epub 2012 Nov 8.
- Gil Gonzalez S, MarquésLópez F, Rigol Ramon P, MestreCortadellas C, Cáceres Palou E, León García A. Two-stage revision of hip prosthesis infection using a hip spacer with stabilising proximal cementation. Hip Int. 2010 May 27;20 (Suppl 7)(S7):128-134. [Epub ahead of print]
- Husby OS, Haugan K, Benum P, Foss OA.A prospective randomisedradiostereometric analysis trial of SmartSet HV and Palacos R bone cements in primary total hip arthroplasty.J OrthopTraumatol. 2010 Mar;11(1):29-35. Epub 2010 Mar 3.
- Joseph TN, Chen AL, Di Cesare PE.Use of antibiotic-impregnated cement in total joint arthroplasty.J Am AcadOrthop Surg. 2003 Jan-Feb;11(1):38-47. Review.
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K.Complications after spacer implantation in the treatment of hip joint infections.Int J Med Sci. 2009 Sep 2;6(5):265-73.
- Kärrholm J, Frech W, Nilsson KG, Snorrason F.Increased metal release from cemented femoral components made of titanium alloy. 19 hip prostheses followed with radiostereometry (RSA).ActaOrthop Scand. 1994 Dec;65(6):599-604.
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J.Prosthetic joint infection risk after TKA in the Medicare population.ClinOrthopRelat Res. 2010 Jan;468(1):52-6. Epub 2009 Aug 8.
- Logoluso N, Champlon C, Melegati G, Dell’oro F, Romanò CL. Gait analysis in patients with a preformed articulated knee spacer. Knee. 2012 Aug;19(4):370-2. Epub 2011 May 7.
- Meyer J, Piller G, Spiegel CA, Hetzel S, Squire M.Vacuum-mixing significantly changes antibiotic elution characteristics of commercially available antibiotic-impregnated bone cements.J Bone Joint Surg Am. 2011 Nov 16;93(22):2049-56.
- Millennium Group Survey, 2011 – Global market for Large Joint Reconstruction
- Moojen DJ, Hentenaar B, Charles Vogely H, Verbout AJ, Castelein RM, Dhert WJ.In vitro release of antibiotics from commercial PMMA beads and articulating hip spacers.J Arthroplasty. 2008 Dec;23(8):1152-6. Epub 2008 Mar 4.
- Mutimer J, Gillespie G, Lovering AM, Porteous AJ.Measurements of in vivo intra-articular gentamicin levels from antibiotic loaded articulating spacers in revision total knee replacement.Knee. 2009 Jan;16(1):39-41. Epub 2008 Sep 10.
- Neumann DR, Hofstaedter T, List C, Dorn U. Two-stage cementless revision of late total hip arthroplasty infection using a premanufactured spacer.J Arthroplasty. 2012 Aug;27(7):1397-401. Epub 2011 Dec 16.
- Nivbrant B, Kärrholm J, Röhrl S, Hassander H, Wesslén B.Bone cement with reduced proportion of monomer in total hip arthroplasty: preclinical evaluation and randomized study of 47 cases with 5 years’ follow-up.ActaOrthop Scand. 2001 Dec;72(6):572-84.
- Norwegian Arthroplasty Register http://nrlweb.ihelse.net/eng/default.htm
- Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J.Prosthetic joint infection risk after total hip arthroplasty in the Medicare population.J Arthroplasty. 2009 Sep;24(6 Suppl):105-9. Epub 2009 Jun 2.
- Pattyn C, De Geest T, Ackerman P, Audenaert E.Preformed gentamicin spacers in two-stage revision hip arthroplasty: functional results and complications.IntOrthop. 2011 Oct;35(10):1471-6. doi: 10.1007/s00264-010-1172-8. Epub 2010 Nov 30.
- Pitto RP, Castelli CC, Ferrari R, Munro J.Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty.IntOrthop. 2005 Oct;29(5):305-8. Epub 2005 Aug 5.
- Pitto RP, Schmidt R. CEMEX® GENTA bone cement in total hip arthroplasty. Clinical outcome and Radiostereoanalysis of 25 hips with 2-year follow-up. Clinical Report, July 4, 2003
- Pitto RP. CEMEX® SYSTEM GENTA bone cement in total hip arthroplasty. Clinical outcome and Radiostereoanalysis . A 5-year follow-up. Clinical Report, April 6, 2007
- Rogers BA, Middleton FR, Shearwood-Porter N, Kinch S, Roques A, Bradley NW, Browne M.Does cyclical loading affect the elution of antibiotics from articulating cement knee spacers?J Bone Joint Surg Br. 2011 Jul;93(7):914-20.
- Romanò CL, Ferrarin M, Rabbuffetti M, Recalcati M, Meani E. Analysis of the gait in patients with hip spacers. In “Infection and local treatment in orthopedic surgery”. Eds . Meani E, Romanò C, Crosby L, Hoffman G – Springer-Verlag, February 2007
- Romanò CL, Romanò D, Albisetti A, Meani E.Preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Long-term results.Hip Int. 2012 Jul-Aug;22 Suppl 8:S46-53.
- Romanò CL, Romanò D, Meani E, Logoluso N, Drago L.Two-stage revision surgery with preformed spacers and cementless implants for septic hip arthritis: a prospective, non-randomized cohort study.BMC Infect Dis. 2011 May 16;11:129.
- Scheerlinck T, Casteleyn PP.The design features of cemented femoral hip implants.J Bone Joint Surg Br. 2006 Nov;88(11):1409-18. Review.
- Söderlund P, Dahl J, Röhrl S, Nivbrant B, Nilsson KG.10-year results of a new low-monomer cement: follow-up of a randomized RSA study.ActaOrthop. 2012 Dec;83(6):604-8. Epub 2012 Nov 1.
- Squire MW, Ludwig BJ, Thompson JR, Jagodzinski J, Hall D, Andes D.Premixed antibiotic bone cement: an in vitro comparison of antimicrobial efficacy.J Arthroplasty. 2008 Sep;23(6 Suppl 1):110-4. Epub 2008 Jul 9.
- Swedish Hip Arthroplasty Register http://www.shpr.se/en/default.aspx
- van de Belt H, Neut D, Uges DR, Schenk W, van Horn JR, van der Mei HC, Busscher HJ.Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release.Biomaterials. 2000 Oct;21(19):1981-7.
- Villa T, Carnelli D. Experimental evaluation of the biomechanical performances of a PMMA-based knee spacer. Knee 2007 Mar;14(2):145-53
- Wan Z, Karim A, Momaya A, Incavo SJ, Mathis KB.Preformed articulating knee spacers in 2-stage total knee revision arthroplasty: minimum 2-year follow-up.J Arthroplasty. 2012 Sep;27(8):1469-73.Epub 2012 Mar 14. Erratum in: J Arthroplasty. 2012 Dec;27(10):1879.