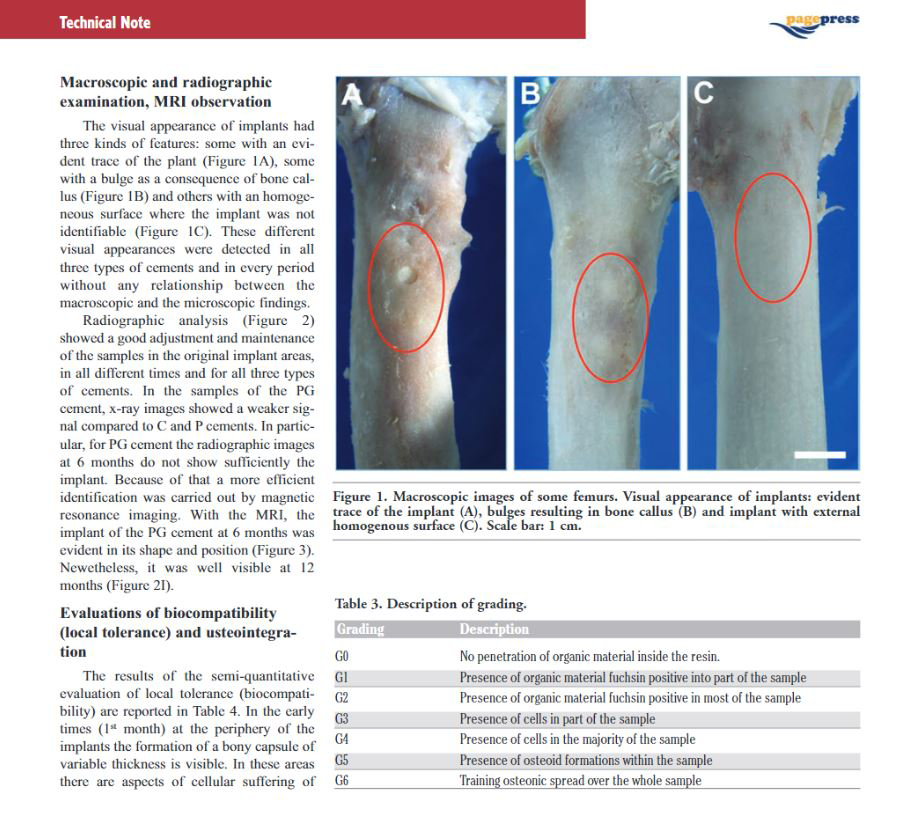
The preformed spacer
2013
Giovanni Calonego
Tecres Scientific Coordinator
What is a spacer, and what is its function?
A spacer is a device made of bone cement (PMMA) and antibiotic resembling the shape of a prosthesis whose aim is to replace temporarily the prosthesis removed due to a septic cause maintaining space, partial mobility and allowing for partial / limited weight-bearing in the period during which infection is eradicated. The function of the antibiotic is to protect the device from bacterial adhesion and release locally antibiotic
Which are the characteristics of a preformed spacer?
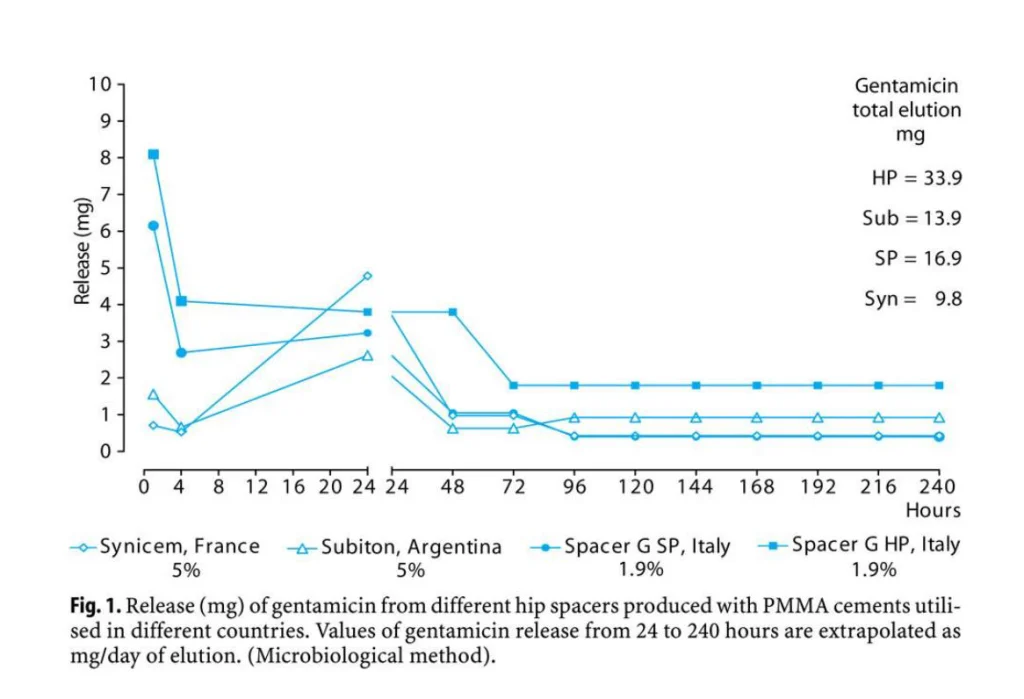
A preformed spacer is an industrially made device with standardized performances, designed to provide safety and effectiveness. Spacers have been designed to be able to replace in complete safety a prosthesis during 6-month period. Mechanical testing (Baleani et al., 2003; Villa et al., 2007) have shown that the performances of the devices are superimposable with the performances of definitive prosthesis in the short-term (6 months). Pharmacological testing has shown that the devices release high amount of antibiotic following implantation (Bertazzoni et al., 2007) and keeps releasing antibiotic over time guaranteeing high concentration of antibiotic in the joint all over the implantation period (Mutimer et al., 2009). Explanted spacer are still able to release limited but effective amount of antibiotics (Bertazzoni et al., 2004).
Why is it important to have reproducible performances?
Peri-prosthetic joint infection is a major complication in orthopaedic surgery. Each infection can be considered as a single case as a number of factors concur to determine the success of the surgical procedure to get rid of the infection. The preformed spacers are industrial product with performances which are not changing over time, and therefore the surgeon and the patient know in advance what to expect.
The production of spacer devices in the theatre cannot guarantee by industrial products which undergo quality control tests prior to being released and have been studied to provide the performances required in this difficult task.
Why the antibiotic Gentamicin has been selected?
Gentamicin is a wide spectrum antibiotic active against Gram positive and Gram negative bacteria. Gentamicin is available in powder form, heat resistant and non-allergenic, has well known side effects and has good elution profile from PMMA.
Why it does exist the spacer with the combination of Gentamicin + Vancomycin (Vancogenx-Space)? When the pathogen is resistant to Gentamicin, and susceptible to Vancomycin the use of Vancogenx is indicated. In such cases, the combination of the antibiotics is synergistic and the antimicrobial effectiveness is enhanced (Bertazzoni et al., 2011; Gallo et al., 2013)
The spacer is made of bone cement and antibiotic. Why the mechanical and pharmacological characteristics are different from those of spacers made in the theatre?
The admixture of antibiotic affects negatively the mechanical performances of bone cement, and the antibiotic release is not directly related to the amount of added antibiotic (Dunne et al., 2007). The intra-operative preparation of spacer cannot guarantee adequate dispersion of the antibiotic in the PMMA mixture and this has a relevant impact both on release and mechanical strength of the device. Unpredictable antibiotic release and mechanical spacer breakage (Jung et al., 2009) are highly related with hand-made spacer devices. Technology may modify porosity of the PMMA matrix in order to achieve a high and prolonged antibiotic release. The concept of dose should be replaced by the concept of release.
How antibiotic is released from PMMA? When the biological liquids come in contact with the antibiotic present in the PMMA, a dissolution process starts: the antibiotic is solubilized, and elute from PMMA matrix leaving a holey structure. The more the PMMA matrix is porous, the more the structure will have holes and this will allow the biological liquids to penetrate and dissolve more antibiotic (van de Belt et al., 2000).
Is the use of the preformed spacer associated with good clinical outcomes?
The preformed spacers have been introduced in the European market at the end of 1996, and started to be marketed in US in 2004. Over the years several papers have been published indicating that excellent results are achieved with the preformed spacer (hip, knee, shoulder). The table below summarizes the most relevant studies. Main outcome is that at a mean follow-up of 46 months in a total of almost 500 patients coming from 12 Centers around the world the average eradication rate have been of 94.9%.