Premixed Antibiotic Bone Cement
Matthew W. Squire, MD,*yBrian J. Ludwig, MD,*yJonathan R. Thompson, BS,z
Jason Jagodzinski, BS,zDerek Hall, BS,zand David Andes, MD§∥
Abstract: After Food and Drug Administration (FDA) approval of premixed antibiotic bone cements (polymethylmethacrylate [PMMA]), these products are being used with increasing frequency during revision and primary hip and knee arthroplasties. To date, no studies have compared the antimicrobial efficacy of more than 2 products directly. Using a 7-day modified Kirby-Bauer assay, we assessed the in vitro antibacterial properties of 5 FDA-approved, commercially available antibiotic PMMAs. Significant differences in antimicrobial activity were noted among the antibiotic PMMA products included in this investigation. Antibacterial activity of all products tested was greatest on day 1 and rapidly diminished thereafter. Results of this investigation suggest that the antibacterial efficacies of premixed antibiotic PMMA products are not equivalent.Key words:premixed antibiotic bone cement, polymethylmethacrylate, total hip anthroplasty, total knee arthroplasty. © 2008 Published by Elsevier Inc
Deep periprosthetic infection after total knee arthroplasty (TKA) or total hip arthroplasty (THA) continues to be a problem. Despite the relatively low incidence of this surgical complication, the sequelae and cost of deep TKA and THA infections are significant. Deep infection after TKA or THA frequently results in the need for multiple surgical interventions, the need for extended duration parenteral antibiotics, and in some cases, compromised function of subsequent revision TKA or THA. The health care costs of reconstructing previously infected TKAs and THAs have been estimated to be approximately 5 times that of a primary TKA or THA[1].
Recently, the Food and Drug Administration (FDA) has approved a number of commercially available premixed
From the *University of Wisconsin Department of Orthopedic Surgery, Madison, Wisconsin;yUniversity of Wisconsin Hospital and
Clinics,Madison, Wisconsin;zUniversity of Wisconsin School of Medicine and Public Health, Health Sciences Learning Center, Madison, Wisconsin;§University of Wisconsin Section of Infectious Disease, Madison, Wisconsin; andOUniversity of Wisconsin Hospital and Clinics, Madison, Wisconsin. Submitted January 31, 2008; accepted March 22, 2008. No benefits or funds were received in support of the study.
Reprint requests: Matthew W. Squire, MD, Department of
Orthopedics and Rehabilitation, K4/7 Clinical Science Center,
600 Highland Ave, Madison, WI 53792-7375.
© 2008 Published by Elsevier Inc.
0883-5403/08/2306-0020$34.00/0
doi:10.1016/j.arth.2008.03.014
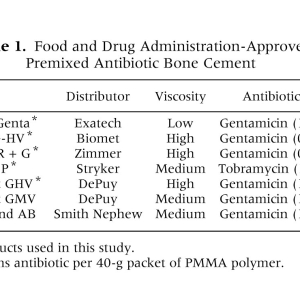
antibiotic bone cement products to aid in the reconstruction of previously infected TKAs and THAs [2](Table 1). The approved use of these products is for the revision of infected TKAs or THAs after 2-stage infection eradication protocol. Some United States surgeons are routinely or selectively using these products off-label to provide additional infection prophylaxis during primary or revision TKAs and THAs. Despite the routine and longstanding use of antibiotic impregnated bone cements in Europe for primary and revision TKA and THA, their routine use in the United States remains controversial[2]. The premixed antibiotic bone cements approved by the FDA differ with respect to viscosity, antibiotic concentration, and type of antibiotic (Table 1). To date, there are few peer-reviewed, nonindustry sponsored studies detailing the antimicrobial efficacy of these products. In addition, there are no studies that compare the antimicrobial properties of more than 2 products directly[3-6]. The purpose of this investigation is to compare the in vitro antimicrobial efficacy of these commercially available premixed antibiotic bone cements such that they can be used in an evidence-based manner.
Methods
Antibiotic bone cements (PMMA) used in this investigation were either donated by the manufacturer or purchased from hospital stock as provided to our surgical institution by the vendor. All PMMA were hand mixed under atmospheric pressure using a standardized mixing vessel and spatula. Mixing order, duration, and frequency were per the manufacturers’ recommendations If no recommendations were provided by the manufacturer, the liquid (monomer) was added to the polymer (powder) and mixed at one revolution per second for 60 seconds. Using a novel process developed in our laboratory, highly standardized PMMA disks were constructed from PMMA without antibiotics (negative control) and from the antibiotic PMMA products included in this investigation (Table 1). A modified Kirby-Bauer assay was then performed using the PMMA disks. Briefly, negative controls and 10 disks per antibiotic PMMA product were plated on Mueller-Hinton solid growth media containing a clinically relevant and virulent Staphylococcus aureus organism (strain 6538P). The growth media was incubated at 37°C for 24 hours at which time the PMMA disks were removed and transferred onto new growth media for 7 consecutive days. To avoid potential bias introduced by PMMA lot, the assay was repeated 3 times for all products using PMMA disks from different lots. The zone of bacterial growth inhibition (ZOI) created by PMMA disks was recorded daily for all disks using 8 megapixel digital photographs. A publicly available image processing program, NIH ImageJ (National Institutes of Health, Bethesda, Md), was then used to measure and quantify the ZOI areas (mm 2). Analysis of variance (ANOVA) was used to test for ZOI differences (i) across time (repeated measures ANOVA) and (ii) across brands within each day (3-way ANOVA). Differences among ZOI means achieving P b .05 were deemed statistically significant.
Results
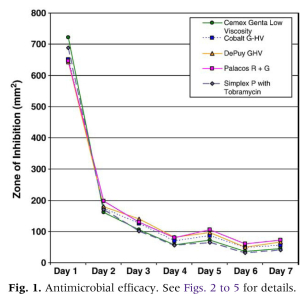
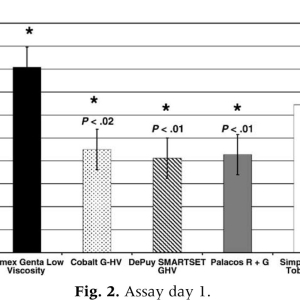
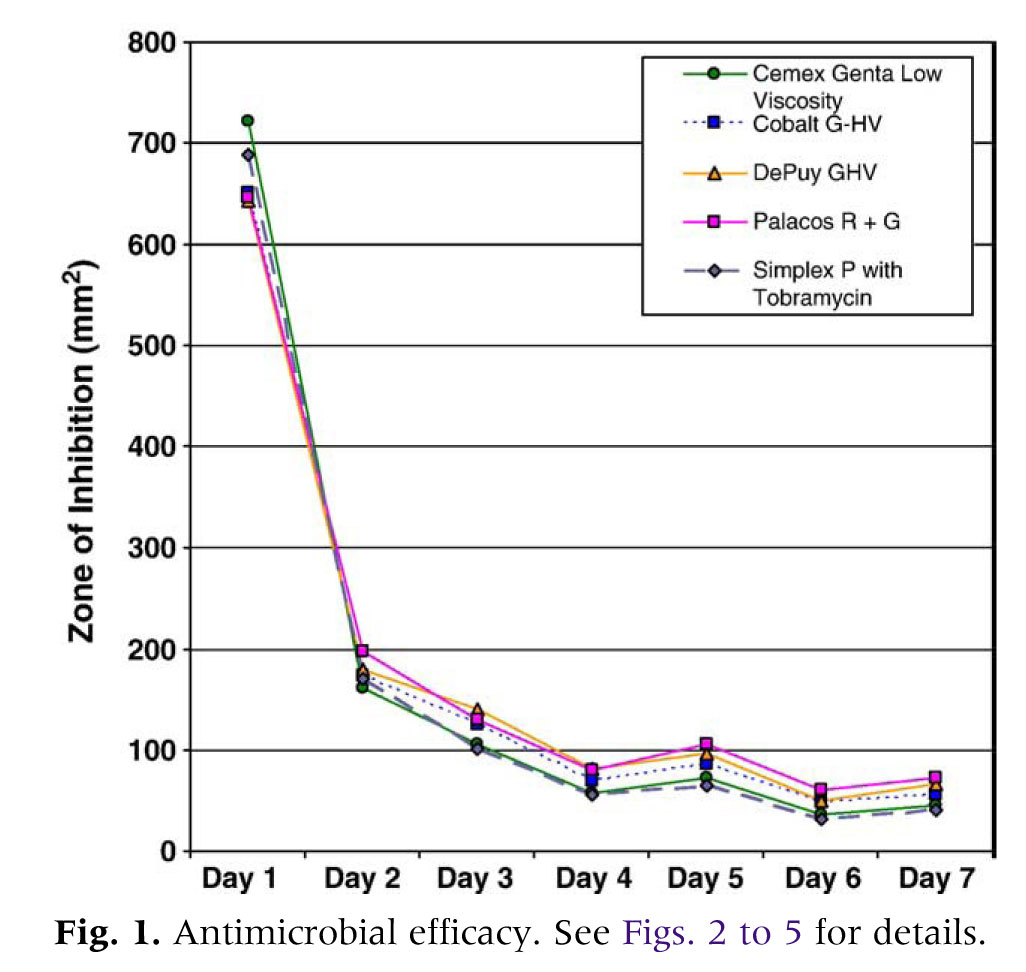
All antibiotic PMMA products assayed produced detectable bacterial growth inhibition for all days of the assay (Fig. 1). By using repeated measures ANOVA, statistically significant declines in the ZOI were observed when successive days of the assay were compared for each brand of bone cement. Three-way ANOVA revealed statistical differences in antibacterial efficacy among the antibiotic bone cements for all days of the assay except for day 3. The PMMA disks without antibiotics did not produce any bacterial growth inhibition. Growth inhibition for all products was greatest on assay day 1 and rapidly declined thereafter. In general, ZOI produced on day 2 of the assay was approximately 30% of that produced on assay day 1 (eg, ZOI for Palacos R + G [Zimmer, Inc, Warsaw, Ind] on assay day 1 was 650 mm 2 and 200 mm 2 on assay day 2). The decline in antimicrobial efficacy noted between day 2 and day 3 of the assay was not as extreme; ZOI on assay day 3 was approximately 70% of that detected on assay day 2. Repeated measures ANOVA performed for all products demonstrated that the ZOI on assay day 1 was significantly greater than that of assay day 2 (Pb.0001), day 2 was greater than day 3 (Pb.0002), and day 3 was greater than day 4 (Pb.0001). On assay day 1 (Fig. 2), Cemex Genta (Exatech, Gainesville, Fla) demonstrated significantly greater bacterial growth inhibition than Palacos R + G (Pb.01),DePuy Smartset GHV (DePuy Orthopaedics, Warsaw, Ind) (Pb.01), and Cobalt G-HV (Biomet, Warsaw, Ind) (Pb 02). Cemex Genta appeared to produce more bacterial growth inhibition than Simplex P with tobramycin (Stryker, Mahwah, NJ); however, this trend did not reach statistical significance. Likewise, Simplex P with tobramycin appeared to have greater antimicrobial efficacy than did Palacos R + G, DePuy Smartset GHV, and Cobalt G-HV, but this trend did not reach statistical significance. Overall, a trend for the low-viscosity and medium-viscosity cements to produce greater bacterial growth inhibition as compared to the high-viscosity cements (Palacos R+G, DePuy Smartset GHV, and CobaltG-HV) was recognized on day 1.
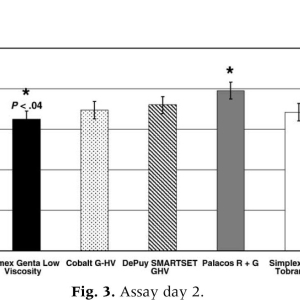
On assay day 2 (Fig. 3), Palaco R + G demonstrated significantly greater antimicrobial efficacy than Cemex Genta (Pb.04). The remaining products (DePuy Smartset GHV, Cobalt G-HV, and Simplex P with tobramycin) produced bacterial growth inhibition that was less than that of Palacos R + G but greater than Cemex Genta. The antimicrobial efficacy of these products did not significantly differ from that of Palacos R + G or Cemex Genta. On assay day 3, no statistical differences were noted among products with respect to antimicrobial efficacy However, the bacterial growth inhibition on this day was greatest for DePuy Smartset GHV and lowest for Simplex P with tobramycin. Despite the lack of statistical significance, a strong trend was observed for high-viscosity products (Palacos R+G, DePuy Smartset GHV, and Cobalt G-HV)to produce a greater degree of bacterial growth inhibition than low-viscosity and medium-viscosity products (Cemex Genta and Simplex P with tobramycin).
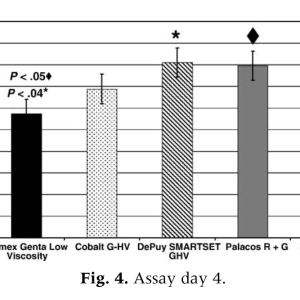
On assay day 4 (Fig. 4), both Palacos R + G and DePuy Smartset GHV produced significantly more growth inhibition than Cemex Genta (Pb.05 andPb.04, respectively) and Simplex P with tobramycin (Pb.03 and Pb.02, respectively). In addition, Cobalt G-HV also appeared to cause more growth inhibition than did Cemex Genta and Simplex P with tobramycin; however, this did not reach statistical significance. On day 4, 2 of the 3 high-viscosity bone cements produced significantly greater bacterial growth inhibition than the lower viscosity products. On assay day 5, Palacos R + G demonstrated significantly more bacterial growth inhibition than either Simplex P with tobramycin (Pb.006) or Cemex Genta (Pb.02). In addition, DePuy Smartset GHV produced antibiotic bone cements for all days of the assay except for day 3. The PMMA disks without antibiotics did not produce any bacterial growth inhibition. Growth inhibition for all products was greatest on assay day 1 and rapidly declined thereafter. In general, ZOI produced on day 2 of the assay was approximately 30% of that produced on assay day 1 (eg, ZOI for Palacos R + G [Zimmer, Inc, Warsaw, Ind] on assay day 1 was 650 mm 2 and 200 mm 2 on assay day 2). The decline in antimicrobial efficacy noted between day 2 and day 3 of the assay was not as extreme; ZOI on assay day 3 was approximately 70% of that detected on assay day 2. Repeated measures ANOVA performed for all products demonstrated that the ZOI on assay day 1 was significantly greater than that of assay day 2 (Pb.0001), day 2 was greater than day 3 (Pb.0002), and day 3 was greater than day 4 (Pb.0001). On assay day 1 (Fig. 2), Cemex Genta (Exatech, Gainesville, Fla) demonstrated significantly greater bacterial growth inhibition than Palacos R + G (Pb.01), DePuy Smartset GHV (DePuy Orthopaedics, Warsaw, Ind) (Pb.01), and Cobalt G-HV (Biomet, Warsaw, Ind) (Pb 02). Cemex Genta appeared to produce more bacterial growth inhibition than Simplex P with tobramycin (Stryker, Mahwah, NJ); however, this trend did not reach statistical significance. Likewise, Simplex P with tobramycin appeared to have greater antimicrobial efficacy than did Palacos R + G, DePuy Smartset GHV, and Cobalt G-HV, but this trend did not reach statistical significance. Overall, a trend for the low-viscosity and medium-viscosity cements to produce greater bacterial growth inhibition as compared to the high-viscosity cements (Palacos R + G, DePuy Smartset GHV, and Cobalt G-HV) was recognized on day 1. On assay day 2 (Fig. 3), Palacos R + G demonstrated significantly greater antimicrobial efficacy than Cemex Genta (Pb.04). The remaining products (DePuy Smartset GHV, Cobalt G-HV, and Simplex P with tobramycin) produced bacterial growth inhibition that was less than that of Palacos R + G but greater than Cemex Genta. The antimicrobial efficacy of these products did not significantly differ from that of Palacos R + G or Cemex Genta.
On assay day 3, no statistical differences were noted among products with respect to antimicrobial efficacy However, the bacterial growth inhibition on this day was greatest for DePuy Smartset GHV and lowest for Simplex P with tobramycin. Despite the lack of statistical significance, a strong trend was observed for high-viscosity products (Palacos R + G, DePuy Smartset GHV, and Cobalt
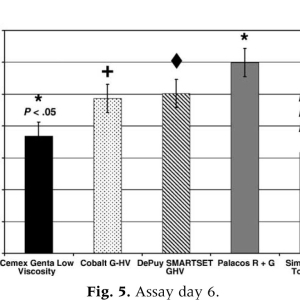
G-HV) to produce a greater degree of bacterial growth inhibition than low-viscosity and medium-viscosity products (Cemex Genta and Simplex P with tobramycin). On assay day 4 (Fig. 4), both Palacos R + G and DePuy Smartset GHV produced significantly more growth inhibition than Cemex Genta (Pb.05 andPb.04, respectively) and Simplex P with tobramycin (Pb.03 and Pb.02, respectively). In addition, Cobalt G-HV also appeared to cause more growth inhibition than did Cemex Genta and Simplex P with tobramycin; however, this did not reach statistical significance. On day 4, 2 of the 3 high-viscosity bone cements produced significantly greater bacterial growth inhibition than the lower viscosity products. On assay day 5, Palacos R + G demonstrated significantly more bacterial growth inhibition than either Simplex P with tobramycin (Pb.006) or Cemex Genta (Pb.02). In addition, DePuy Smartset GHV produced significantly greater growth inhibition than Simplex P with tobramycin (Pb.03). The growth inhibition of Cobalt G-HV appeared to be greater than both Simplex P with tobramycin and Cemex Genta; however, again, this trend was not significant. In general, high-viscosity bone cements again appeared to demonstrate greater antimicrobial efficacy than did the lower viscosity cements. On assay day 6 (Fig. 5), all high-viscosity cements produced statistically greater antimicrobial efficacy than the lower viscosity cements. Palacos R + G was statistically superior to both Cemex Genta (Pb.05) and Simplex P with tobramycin (Pb.002). DePuy Smartset GHV (Pb.02) and Cobalt G-HV (Pb.02) both demonstrated significantly greater bacterial growth inhibition than Simplex P with tobramycin. Although DePuy Smartset GHV and Cobalt G-HV appeared to produce more bacterial growth inhibition than Cemex Genta, this trend did not reach statistical significance. No significant differences were noted between the high-viscosity PMMA products (Palacos R + G, DePuy Smartset GHV, and Cobalt G-HV). On the last day of the assay, all high-viscosity cements again produced greater bacterial growth inhibition as compared to the low-viscosity and medium-viscosity cements. Palacos R + G was statistically superior in its antimicrobial efficacy than Simplex P with tobramycin (Pb .007) and Cemex Genta (Pb.02). DePuy Smartset GHV demonstrated statistically greater bacterial growth inhibition than both Simplex (Pb.02) and Cemex Genta (Pb.05). Cobalt G-HV again demonstrated a trend of greater bacterial growth inhibition as compared to both Cemex Genta and Simplex P with tobramycin, but this did not reach statistical significance.
Discussion
All commercially available premixed antibiotic bone cements assayed in this in vitro investigation produced significant bacterial growth inhibition. Antimicrobial efficacy of all products was greatest on assay day 1 and decreased in a rapid and statistically significant manner during the first 4 days of this investigation. This rapid and significant decline in bacterial growth inhibition indicates that the maximal clinical antibacterial effect of premixed antibiotic bone cements occurs in the early postoperative period.
In general, low-viscosity and medium-viscosity products produced the greatest early (assay day one) bacterial growth inhibition. However, by assay day 2, high-viscosity products appeared to produce superior antimicrobial efficacy as compared to the lower viscosity products. Throughout the remainder of the assay (days 3-7), all high-viscosity bone cements demonstrated a trend for superiority or were significantly superior with respect to bacterial growth inhibition when compared to the lower viscosity products.
Both intensity and duration of bacterial growth inhibition are important theoretical considerations when determining which premixed antibiotic bone cement product is preferable during revision or primary joint reconstruction [2]. The use of antibiotic bone cement has been implicated in the promotion of bacterial resistance presumably because of long-term subtherapeutic levels of antibiotic eluted from the cement[2]. The PMMA products that have an intense initial burst of antibiotic elution and later elute little or no antibiotics may minimize the promotion of antibacterial resistance. Products with the greatest degree of early bacterial growth inhibition would likely have the greatest ability to kill or inhibit the growth of surgical wound bacterial contaminants immediately after TKA or THA as these bacteria are still in the planktonic phase and highly susceptible to antibiotics.
The results of this investigation indicate that the maximal bacterial growth inhibition for all antibiotic PMMA products tested occurs on assay days 1 and 2 (Fig. 1). Therefore, it is likely that optimal antibiotic accumulation in the wound hematoma would occur if wound drains were avoided or their duration of use minimized. However, some surgeons prefer and some clinical scenarios require the use of wound drainage systems. If wound drains are to be used, PMMA products that elute significant concentrations of antibiotic for a longer duration would be preferable as they would likely result in the greatest antibiotic accumulation within the wound environment.
This study has the inherent weaknesses of any in vitro study attempting to model a complex in vivo system. It is unknown whether the significant differences in antimicrobial efficacy noted among products compared in this study are clinically relevant. It is possible that this direct diffusion model (Kirby-Bauer assay) does not adequately approximate the wound hematoma environment and that in vitro elution studies using physiologic solutions or in vivo studies of wound drainage fluids might yield different results. In addition, this study does not assess the effect of commonly used intraoperative cement preparation techniques (eg, vacuum mixing) that might also change the in vitro and in vivo antimicrobial behavior of these bone cement products[7]. The strength of this study is that it assesses the antimicrobial efficacy of 5 commercially available antibiotic bone cement products in an unbiased and nonindustry sponsored study.
This in vitro comparison indicates that these products vary significantly with regard to both the intensity of their antimicrobial effect as well as the duration of their antimicrobial activity. Further studies will be needed to define the effect that intraoperative preparation (eg, vacuum mixing vs mixing under atmospheric pressure) has on these antibiotic bone cement products. It is possible that one antibiotic cement product is not preferable for all clinical situations. Retrospective studies indicate that some varieties of these “premixed” antibiotic bone cements perform quite well in vivo[8-10]. Unfortunately, there are no clinical trials to date that compare the efficacy of these products in vivo, and some products have been used for such a short period that little follow-up data exist concerning their clinical performance. Therefore, at this time, we recommend basing antibiotic product choice on prior performance of the available premixed antibiotic bone cements.
References
- Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am 2005;87:1746.
- Jiranek WA, Hanssen AD, Greenwald AS. Antibioticloaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006;88: 2487.
- Sterling GJ, Crawford S, Potter JH, et al. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003;85:646.
- Lewis G, Janna S. The in vitro elution of gentamicin sulfate from a commercially available gentamicinloaded acrylic bone cement, VersaBond AB. J Biomed Mater Res B Appl Biomater 2004;71:77.
- Davies JP, O’Connor DO, Burke DW, et al. Influence of antibiotic impregnation on the fatigue life of Simplex P and Palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res 1989;23:379.
- Dall GF, Simpson PM, Breusch SJ. In vitro comparison of Refobacin-Palacos R with Refobacin Bone Cement and Palacos R + G. Acta Orthop 2007;78:404.
- Neut D, van de BH, van Horn JR, et al. The effect of mixing on gentamicin release from polymethylmethacrylate bone cement. Acta Orthop 2003;74:670.
- Engesaeter LB, Lie SA, Espehaug B, et al. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74: 644.
- Havelin LI, Espehaug B, Vollset SE, et al. The effect of the type of cement on early revision of Charnley total hip prostheses. A review of eight thousand five hundred and seventy-nine primary arthroplasties from the Norwegian Arthroplasty Register. J Bone Joint Surg Am 1995;77:1543.
- Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand 2000;71:111.